编者按:
我们昨天的文章分享了发表在 Microorganism 杂志上关于益生菌与肥胖综述的上半部分,主要以微生物组为核心,阐述了胖代谢机制的研究:万字强文:深度剖析益生菌与肥胖(上)
今天,我们继续分享该综述的下半部分:主要阐述了不同饮食模式对肥胖人群的影响,以及益生菌治疗肥胖的未来方向等。
以下为下半部分的编译内容:
其它因素引起的肥胖
肥胖增加了患慢性肾脏疾病(CKD)的可能性,还提高了发展为末期肾病(ESRD)的可能性,而益生菌可以减少某些尿毒症毒素的产生。
由 Niwa 提出的蛋白代谢假说中,肾小管中的有机阴离子转运体可以吸收由肠道微生物产生的毒素109。
比如,鼠乳杆菌(Lactobacillus murinus)可以阻止高盐饮食小鼠的高血压症状的发展113,114。
Ichii 等人发现在小鼠足细胞中的脂多糖可以引起促炎症现象、降低足细胞特异性的基因表达并且降低细胞活性115。
在末期肾病患者中大约有 190 种微生物操作分类单元(OUT),与健康状态时相比要更为丰富116;在慢性肾脏疾病患者中,发现了普雷沃氏菌科和乳杆菌科(均为天然结肠微生物)降低,而肠球菌和肠杆菌增长了 100 倍116。
同时给患有自发性狼疮肾炎的混合淋巴细胞反应(MLR)的小鼠添加口服补冲剂,可以降低肠道通透性,减少系统性炎症反应,从而增强肾脏功能和提高整体存活率。
另一项研究发现,在使用嗜酸乳杆菌治疗自发性 5/6 高血压肾切除大鼠(典型的 CKD 模型)时会减轻肾脏损伤,并且会加强胃肠道屏障功能、减缓内部肿胀并且积累尿毒症毒素117。
当用多种细菌治疗乙酰氨基酚诱导的尿毒症大鼠和铬诱导的氧化应激大鼠时,研究发现,脂质过氧化作用减弱,而抗氧化相关的酶类(过氧化物歧化酶和水解酶)的活性发生了提高118-120;另外,减缓氧胁迫可以减缓肾坏死119,121,122。
益生菌可以保护肾脏,避免其被损伤,具体也就是可以通过减少肿胀、凋亡和氧化应激来减少功能障碍。

某些实验现象可能会出现不一致的结果,这可能是由于实验过程中所用到的细菌不同、患者群体不同以及动物模型的不同所造成的。
肥胖通过改变菌群组成而引起肠道环境变化,这可能是造成肾脏损伤的原因之一。
实验发现,特定的个人和微生物群特征可以预测特定的葡萄糖反应,这意味着人们有可能可以通过饮食、益生元和益生菌干预进行个性化的微生物组调控123。
用益生菌调节肠道菌群平衡可能是肥胖患者改善肾功能的一种较为合适的选择。
FAO 和 WHO 将益生元定义为:不可消化的食物,它可以促进特定的肠道菌的活性进而对宿主产生有益的影响124。
益生元主要为不可消化和水解的碳水化合物,比如果糖寡糖(FOSs)、半乳糖寡糖(GOSs)、大豆寡糖、环糊精、菊粉、葡萄糖寡糖、木糖寡糖、乳果糖、乳蔗糖和异麦芽寡糖等,这些碳水化合物具有到达人体肠道远端的能力125。
已有实验证明,益生元可以对肠道菌群有促进作用,它可以促进肥胖动物肠道内的乳杆菌和双歧杆菌的生长126。
母乳是牛奶低聚糖(可能是益生元)的丰富来源,它能促进有益菌(拟杆菌和双歧杆菌)的生长,并抑制大肠杆菌、空肠弯曲杆菌和幽门螺杆菌等病原体的粘附127。

多项试验都发现,低聚果糖在治疗肥胖和糖尿病小鼠时会引起变化,该变化与肠内分泌细胞生长加速、葡萄糖稳态和瘦素敏感性有关128,129。
这些变化还与细胞内胰高血糖素样肽-2(GLP-2)的高产量有关,GLP-2 与肠道通透性相关,可以减少肥胖相关性胃炎和心血管疾病。
基于此, Everard 等在用低聚果糖治疗动物时发现了非肥胖代谢表型,具体表现为降低甘油三酯浓度、脂肪组织和肌肉脂质浸润128。
关于鼠李糖乳杆菌 GG 治疗儿童肥胖相关的非酒精性脂肪肝的积极影响,现在已有大量的研究支持。
在多数临床研究中,益生元可以减少肝脏组织中甘油三酯和/或胆固醇的积累,即减少脂肪变性。这一研究现象是很有意义的,因为 25%至 75%的肥胖者患有非酒精性脂肪肝。
尽管能量摄入功能是一个问题,但研究并未发现长期补充益生元,如餐前菊粉和低聚半乳糖或短期果糖低聚糖,有任何影响130。
其它研究表明,非肥胖和肥胖个体的寡果糖或菊粉补充至少在 2 周内,可以减少他们的总能量摄入量130-133。
对益生元的实验发现,益生元可以对体重、腰围、BMI、脂质分布、脂肪沉积和慢性炎症状态具有明显的有益调控作用,这可能成为肥胖和相关代谢紊乱的替代治疗方法123。
肥胖与免疫系统的关系及母婴传递
胃肠道黏膜吸收的增加(如肠漏综合征),特别是与免疫变化相关的,可以引起对肠道的重大伤害。具体来讲,会造成细菌、毒素、营养物质和代谢垃圾从肠道中渗漏到血液中,当这些毒素进入肝脏中时会引起严重的自身免疫反应134。
Moya-Pérez 等人在鼠模型中发现,具有假链状双歧杆菌 CECT7765 的肠道生态系统调节了高脂饮食(HFD)诱导的免疫细胞浸润以及肠道和外周炎症,并改善了肥胖相关的代谢功能障碍135。
研究还发现,双歧杆菌的抗炎症效应与 B 淋巴细胞相关的先天免疫功能和适应性免疫功能相关。
Zhang 等人发现,通过为仔猪接种两个洛德乳杆菌菌株 ZJ617 和 ZJ615,发现 ZJ617 具有较强的黏附作用,而 ZJ615 的黏附作用较弱136。他们还进一步研究了不同粘附能力菌株的免疫调节作用。
Kowalska 等人首先在大鼠中概述了高脂饮食对瘦素水平的研究,并通过实验进行了验证137-140。
研究发现,与对照组大鼠相比,高脂饮食能够引起的瘦素水平升高141,且在大鼠中,不论体重是否增加,高脂饮食均能升高瘦素水平142。这一现象的原因还未被阐明。
除此之外,还有一些其它因素共同起作用,如身体脂肪含量,可以影响免疫应答,但在不同年龄段的人群中存在显着差异,身体脂肪含量的升高在老人和年轻人群中产生的不良反应是不相同的。
研究表明,当 BMI 达到 25(通常被认定为肥胖)的 65 岁以上老年人群生存获益143,144。
除了脂肪组织和免疫细胞之外,其它的脂肪细胞或者参与炎症的促炎因子也会参与到免疫功能中144,146 。
由于益生菌在肥胖治疗中的免疫调节作用,研究者认为在饮食中添加益生菌是对健康有益的。
在近几十年中,产前和产后变化(例如早产和低出生体重,妊娠糖尿病,妊娠期体重增加过多和配方奶喂养)与儿童肥胖的发生率上升息息相关147-150。而且这些患病风险因素在发展中国家急剧增高151-153。
妊娠期糖尿病(Gestational diabetes mellitus,GDM)增加了婴儿肥胖和巨婴的风险。长期妊娠期糖尿病与儿童肥胖和婴儿代谢相关154,155。
Boyle 等人发现父母肥胖会增加脐带间充质的分化能力,造成婴儿肥胖156。儿童肥胖会增加其成人时肥胖的风险,并且会增加多种代谢疾病、糖尿病和心血管疾病的风险157。
肠道微生物组是悉生小鼠(一种用已知菌培养的小鼠)测序技术中的新环境因素。肠道微生物组可以通过影响能量平衡、必需和非必需食物摄取、炎症和肠道阻滞功能的调节信号影响整个机体的代谢,从而刺激体重的增加。
肠道微生物是人体中的一个特殊的实体,它具有比宿主更加庞大的基因组和基因池。
在肥胖和糖尿病之间发现存有新联系,即肠道微生物在肠道组织外(如脂肪组织中)的广泛的生理功能158,159。因此肠道菌群在肥胖和糖尿病的病理中发挥重要作用。
利用无菌小鼠发现,微生物组可能通过调控宿主代谢使小鼠免于饮食诱导肥胖154,157,159。
动物研究表明,怀孕和哺乳期的益生菌可以减少母体高脂饮食诱导的与母体肥胖相关的饮食程序,说明改变双亲的肠道微生物组可能加强父母与婴儿之间的代谢联系160。
Vähämiko 等人在对人体的实验中发现,在怀孕期间补充益生菌可能影响母婴的与肥胖相关的启动子和基因的 DNA 甲基化程度161。
Everard 等人的实验表明,L. rhamnosus GG 对婴儿的影响可以从出生前一个月开始并持续到 6 个月大,具体为通过减少婴儿非正常的体重增加从而改变儿童的发育模式。
使用益生菌可能是对妊娠期糖尿病的一个很有前景的预防或治疗方法。益生菌可以治疗微生物组失调,且使用益生菌已经成为一种提高早产儿健康的有效的干预方式。
基于饮食的肠道菌群控制
饮食在预防肥胖中是一个重要的健康因素,并且与调节肠道菌群密切相关。众多研究表明遗传性肥胖的易感性与肥胖环境(饮食重大变化影响肠道微生物、不运动、久坐的生活方式)相关163。
目前有许多常见的饮食方法,包括标准饮食和西式饮食。益生菌对人体健康的影响、新的益生菌菌株的研究是目前较为流行的研究课题。后续的内容将展示益生菌补充剂如何帮助肥胖病人降低体重并改善肠道菌群。
饮食在肠道菌群的组成方面和肠型的决定方面有着重要的作用164,165。Arumugam 等人称,肠型主要分为 3 类,分别以普雷沃菌属,拟杆菌属和胃瘤球菌属为优势种,肠型的分类不完全依据地域起源165。
特定饮食对肠型改变的影响有:拟杆菌属的改变与高蛋白和动物脂肪饮食相关,而普雷沃菌属的变化与高碳水化合物饮食的摄取相关(图 3)。
Zimmer 等认为,杂食者的肠道菌群丰富度要比素食者的肠道菌群丰富度高166。
实验结果显示,在素食者的肠道微生物中,肠杆菌属,拟杆菌属,双歧杆菌属这些微生物所占的比例要比杂食者的占比少。科水平上肠杆菌科,属水平上克雷伯氏菌属、肠杆菌属、柠檬酸杆菌属和梭菌属,这些菌在二者之间并没有差别。
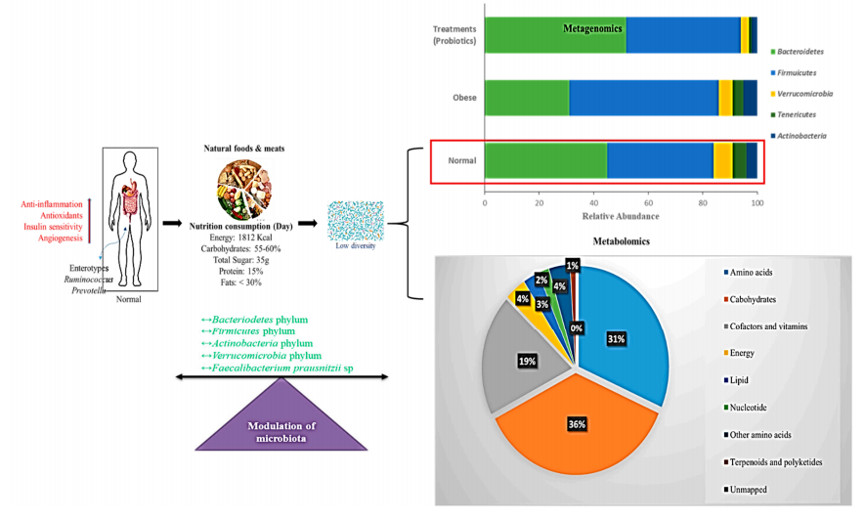
图 3. 正常饮食的调节肠道菌群示意图。
正常饮食、营养、能量摄入和微生物组调节之间的相关作用。条形图代表了宏基因组门水平上的结果。饼图表示了正常饮食代谢影响的百分比。红色箭头表示活性增强。
在许多国家,西方化、城市化和工业化的进程导致了久坐的生活方式、高脂肪高热量的饮食习惯100,167,168。脂肪是饮食的重要部分,是肠道菌群生长和产生短链脂肪酸的底物169,170。
脂肪含量高的食物比如鱼油(ω-3 多不饱和脂肪酸)和猪脂肪(猪油、主要为饱和脂肪酸)对肠道菌群的调控有重要的影响,猪脂肪对拟干菌门和变形菌门有促进增强作用,而鱼油可以促进放线菌门171,172。
大量研究报道肥胖影响特定的菌门,能影响人体和啮齿动物的硬壁菌门和拟杆菌门的比率。
在消瘦人群中,微生物组的差异与粪便的能量损失相关173。当 150 kcal 的收集能量增加 20%时,相应的拟杆菌的数量会降低。据报道,肥胖人群的拟杆菌的相对比例要低于消瘦人群174。
肥胖患者肠道微生物组中硬壁菌门数量的增加,有助于从西式饮食中的热量获取,从而促进更好的卡路里的吸收和体重增加170。
此外,在遗传或饮食诱导的肥胖小鼠和大鼠中,已报道了在 HFD 的对照组中硬壁菌门和拟杆菌门的比例增加107,175。
近期的实验证明西式饮食中急剧增加的脂肪会影响肠道反应信号的调控,导致能量摄入,脂肪积累和炎症176。西式饮食会增加硬壁菌门的数量和脂肪并减少肠道菌群多样性(图 4)。
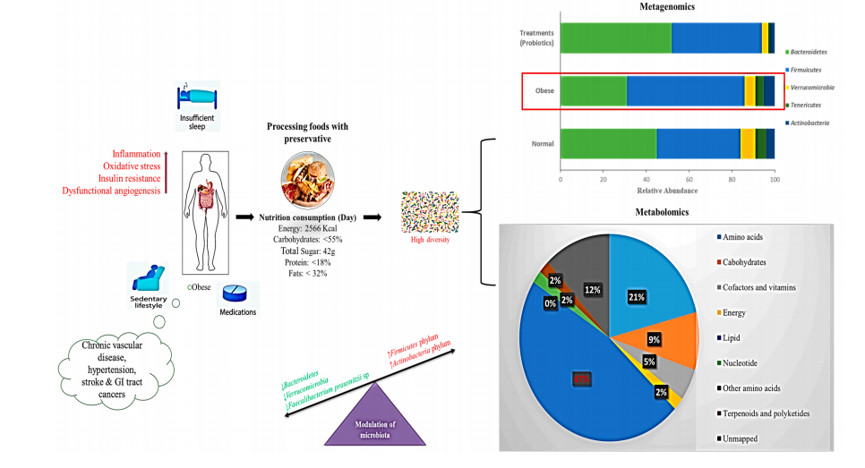
图 4. 西式饮食的调节肠道菌群示意图。
西式饮食、营养、能量摄入和微生物组调节之间的相关作用。条形图代表了宏基因组门水平的结果。饼图表示了西式饮食代谢影响的百分比。红色箭头表示活性增强。
之前许多研究发现,益生菌可以减少脂肪生成、缓解炎症反应并降低体重(图 5)。
特别的,鼠李糖乳杆菌 GG 菌株已经用于肥胖的研究中177,178,鼠李糖乳杆菌 GG 代替 HFD 治疗通过增强脂联素的分泌来抑制高脂饮食小鼠的肥胖。脂联素可以使动物免受胰岛素抗性并缓解脂肪肝179。
另外,在 HFD 小鼠中鼠李糖乳杆菌 GG 产生的胞外多糖可以减少脂肪和脂肪垫的形成,同时通过表达 Toll 样受体 2 缓解炎症178。
当使用含有 14 种(包含双歧杆菌、乳球菌和丙酸杆菌)混合益生菌制剂短期治疗 Wistar 大鼠后,其全身和内脏脂肪组织明显减少,并增强了对胰岛素的敏感性180。
一项涉及 49 个超重和肥胖成人的临床健康研究结果证明了艾克曼菌的丰度与代谢健康具有一定的联系。拥有更高的基因多样性和艾克曼菌的人确实处于更为健康的代谢状况,比如空腹血浆、甘油三酯和身体脂肪分布表现更好181。
通过使用巴氏灭菌失活的艾克曼菌治疗,发现其可以抑制脂肪量增加,胰岛素抗性和血脂异常182,183。这种现象可能是由于 Toll 样受体 2 和巴氏杀菌过程均与艾克曼菌细胞壁上的某种蛋白之间具有相关作用182。
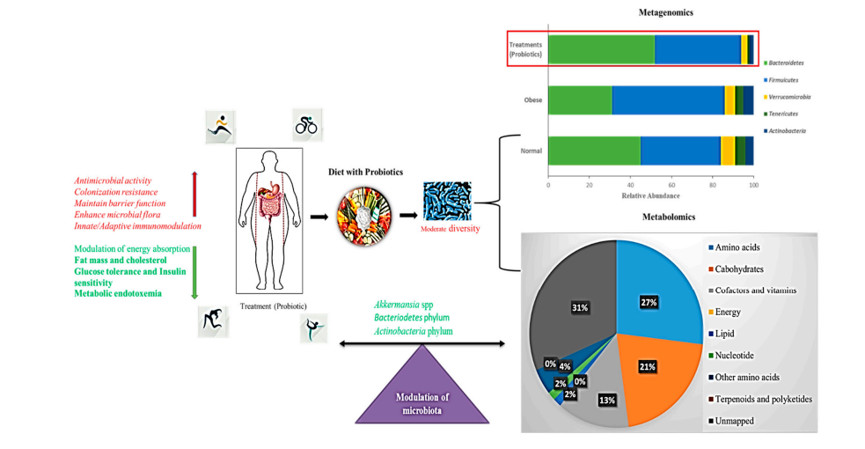
图 5. 益生菌补充的调节肠道菌群示意图。
条形图代表了宏基因组门水平的结果。饼图表示了补充益生菌的西式饮食代谢影响的百分比。红色箭头表示活性增强,绿色表示活性减弱。
艾克曼菌对内毒素血症和流化脂肪的缓解作用表明了益生菌在治疗肥胖方面的应用价值160,162,181。
对肠道微生物宏基因组分析发现,与脂肪、蛋白代谢、碳水化合物代谢相关的 67 条代谢通路均受到了饮食干预的影响,表明了饮食对肠道微生物组代谢的影响。
未来治疗肥胖的方向
粪便菌群移植(Fecal microbiota transplantation,FMT)是将粪便微生物组从特定的供体转移到受体中,是调控肠道菌群较为简单的方法。在多种尖端的研究中,粪菌移植中的受体一般是无菌小鼠。
粪菌移植作为一种创新有效安全的方法,经常在临床中作为治疗艰难梭菌感染的手段184。由于其菌群组成的未知性和复杂性,粪菌移植需要完全按照国际益生菌与益生元科学协会(ISAPP)的指导进行181。
近期,有研究粪菌移植调节肠道菌群并治疗肥胖和代谢紊乱有效性的相关报道发布。Gough 等人发现在 27 项研究中,粪菌移植可以对艰难梭菌感染患者产生积极影响185。粪菌移植可以消除 92%的艰难梭菌感染病例181。
目前有 8 项使用粪菌移植治疗肥胖的注册实验186。
合生元对肠道和宿主的健康要比单独服用益生元或者益生菌更有效果,因为合生元可以给益生菌提供益生元,促进益生菌在肠道内的存活和生长187。合生元已经证明在改善肠道微生物组组成方面是有效的188。
在一个 12 周的研究中,Roller 等人发现使用富含低聚果糖的菊粉、鼠李糖乳杆菌 GG 和动物双歧杆菌 Bb12 后分别可以减少 16%、18%和 31%的产气荚膜梭状芽胞杆菌(Clostridium perfringens)的死亡189。体外研究表明,合生元在调节肠道菌群方面要比益生元和益生菌更有效190。
对肠道微生物代谢组的研究,毫无疑问可以帮助探索肠道微生物、微生物-宿主互作的代谢途径176,191。但是在研究人员想要研究肠道微生物在代谢组方面的代谢调控和与之相关的特定细菌时,面临了许多困难。
宏转录组和宏基因组分析是肠道微生物组方面有效的分析手段192。近期 Sridharan 等人使用基于质谱(MS)的代谢组学开发了基于基因组注释数据的代谢网络模型,用于检测 26 种宏基因组的代谢物193。
目前代谢产物研究最常见的方法是质谱和质子核磁共振(1HNMR)光谱80-82。1HNMR 可以产生生物液体样本(血液、细胞、尿液)的可重复且可靠的代谢组数据,而且需要的样本量较少。
相反,质谱更加灵敏可以检测到浓度显著减少的代谢产物。为了增强分辨率,质谱经常同色谱和气相色谱联用83。
将代谢组学同其它组学结合,比如宏蛋白组、宏基因组和宏转录组,可以加深我们对肠道微生物复杂的生物合成的理解。这样的结合研究可以给肥胖的诊断、优化私人订制医疗和提高单个饮食补充剂有效性等方面带来贡献。在未来,仍有众多肠道微生物的代谢问题等待解决。
结论
本篇综述的目的在于汇总调查使用益生菌治疗肥胖的文献资料。益生菌在动物和人体的研究中发挥了降低体重的作用,并且也发现一些抗肥胖的机制。
早前,微生物群落对代谢调控、疾病和遗传紊乱方面的作用还是一个未知的领域。目前微生物在胆盐、SCFAs、代谢性内毒素血症和肥胖等的调控作用已经在多种分子测序手段如宏基因组、宏代谢组下逐渐被阐明。
然而益生菌的特异性使得确定益生菌作用机制的特定方式变得困难。对人体的研究需要利用宏基因组和宏代谢组来发现更加微小的差异,并探索益生菌用于治疗肥胖和代谢疾病的潜能。
预临床模型有望引导独特且性价比高的益生菌菌株的开发,这将需要在未来几年内完成大量临床研究,以确定它们是否适合人类食用。
使用益生菌、益生元或合生元甚至于粪菌移植来恢复或者调节肠道菌群的组成是预防或治疗肥胖的一种潜在的方法。
然而目前存在的一个问题是用于治疗的益生菌的剂量还没有确定。尽管有大量的实验动物模型,但是不同的给药方法可能会影响到先前研究的结果和结论。
我们认为,按照目前的方向一直前行,可能关于管理肥胖及其相关的代谢缺陷方面的策略将会迎来光明的前景。
参考文献:
(滑动下方文字查看)
1. World Health Organization. Overweightand Obesity. Available online:http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight(accessed on 25 September 2018).
2. Van Baal, P.H.; Hoogenveen, R.T.; deWit, A.G.; Boshuizen, H.C. Estimating health-adjusted life expectancyconditional on risk factors: Results for smoking and obesity. Popul. HealthMetrics 2006, 4, 14.
3. Nascimento Ferreira, M.V.;Rendo-Urteaga, T.; De Moraes, A.C.; Moreno, L.A.; Carvalho, H.B. AbdominalObesity in Children: The Role of Physical Activity, Sedentary Behavior, andSleep Time. In Nutrition in the Prevention and Treatment of Abdominal Obesity;Academic Press: Cambridge, MA, USA, 2019; pp. 81–94. [Google Scholar]
4. FAO; WHO. Guidelines for the Evaluationof Probiotics in Foods. Report of a Joint FAO/WHO Working Group on DraftingGuidelines for the Evaluation of Probiotics in Food; FAO: Rome, Italy; WHO:Geneva, Switzerland, 2002. [Google Scholar]
5. Rajilić-Stojanović, M.; De Vos, W.M. Thefirst 1000 cultured species of the human gastrointestinal microbiota. FEMSMicrobiol. Rev. 2014, 38, 996–1047.
6. Blandino, G.; Inturri, R.; Lazzara, F.;Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus.Diabetes Metab. 2016, 42, 303–315.
7. Gill, H.; Prasad, J. Probiotics,immunomodulation, and health benefits. Adv. Exp. Med. Biol. 2008, 206, 423–454.[Google Scholar]
8. Ashraf, R.; Shah, N.P. Immune SystemStimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54,938–956.
9. Kang, J.-H.; Yun, S.-I.; Park, M.-H.;Park, J.-H.; Jeong, S.-Y.; Park, H.-O. Anti-Obesity Effect of Lactobacillusgasseri BNR17 in High-Sucrose Diet-Induced Obese Mice. PLoS ONE 2013, 8,e54617.
10. Park, Y.H.; Kim, J.G.; Shin, Y.W.; Kim,H.S.; Kim, Y.-J.; Chun, T.; Kim, S.H.; Whang, K.Y. Effects of Lactobacillusacidophilus 43121 and a mixture of Lactobacillus casei and Bifidobacteriumlongum on the serum cholesterol level and fecal sterol excretion inhypercholesterolemia-induced pigs. Biosci. Biotechnol. Biochem. 2008, 72,595–600.
11. Sharma, P.; Bhardwaj, P.; Singh, R.Administration of Lactobacillus casei and Bifidobacterium bifidum AmelioratedHyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J.Prev. Med. 2016, 7, 102. [Google Scholar]
12. So, J.-S.; Kwon, H.-K.; Lee, C.-G.; Yi,H.-J.; Park, J.-A.; Lim, S.-Y.; Hwang, K.-C.; Jeon, Y.H.; Im, S.-H.Lactobacillus casei suppresses experimental arthritis by down-regulating Thelper 1 effector functions. Mol. Immunol. 2008, 45, 2690–2699.
13. Larsen, N.; Vogensen, F.K.; Berg,F.W.J.V.D.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Abu Al-Soud, W.;Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults withType 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085.
14. Kong, Y.; He, M.; McAlister, T.;Seviour, R.; Forster, R. Quantitative Fluorescence In Situ Hybridization ofMicrobial Communities in the Rumens of Cattle Fed Different Diets. Appl.Environ. Microbiol. 2010, 76, 6933–6938.
15. Barrett, E.; Ross, R.; O’Toole, P.;Fitzgerald, G.; Stanton, C. γ-Aminobutyric acid production by culturablebacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417.
16. Khalili, L.; Alipour, B.; Jafar-Abadi,M.A.; Faraji, I.; Hassanalilou, T.; Abbasi, M.M.; Vaghef-Mehrabany, E.; Sani,M.A. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A RandomizedControlled Trial. Iran. Biomed. J. 2019, 23, 68–77.
17. Sabico, S.; Al-Mashharawi, A.;Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ansari, M.G.; Masoud,M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probioticssupplementation in endotoxemic, inflammatory and cardiometabolic status of T2DMpatients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr.2018, 38, 1563–1569.
18. Hu, C.; Wong, F.S.; Wen, L. Type 1diabetes and gut microbiota: Friend or foe? Pharmacol. Res. 2015, 98, 9–15.
19. Ljungberg, M.; Korpela, R.; Ilonen, J.;Ludvigsson, J.; Vaarala, O. Probiotics for the Prevention of Beta CellAutoimmunity in Children at Genetic Risk of Type 1 Diabetes—The PRODIA Study.Ann. N. Y. Acad. Sci. 2006, 1079, 360–364.
20. Hartstra, A.V.; Bouter, K.E.; Bäckhed,F.; Nieuwdorp, M. Insights into the role of the microbiome in obesity and type2 diabetes. Diabetes Care 2015, 38, 159–165.
21. Grover, S.; Rashmi, H.M.; Srivastava,A.K.; Batish, V.K. Probiotics for human health—New innovations and emergingtrends. Gut Pathog. 2012, 4, 15.
22. Szulinska, M.; Łoniewski, I.; VanHemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of MultispeciesProbiotic Supplementation on the Lipopolysaccharide (LPS) Level andCardiometabolic Profile in Obese Postmenopausal Women: A 12-Week RandomizedClinical Trial. Nutrients 2018, 10, 773.
23. Chan, P.A.; Robinette, A.; Montgomery,M.; Almonte, A.; Cu-Uvin, S.; Lonks, J.R.; Chapin, K.C.; Kojic, E.M.; Hardy,E.J. Extragenital Infections Caused by Chlamydia trachomatis and Neisseriagonorrhoeae: A Review of the Literature. Infect. Dis. Obstet. Gynecol. 2016,2016, 1–17.
24. Le Barz, M.; Anhê, F.F.; Varin, T.V.;Desjardins, Y.; Levy, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics asComplementary Treatment for Metabolic Disorders. Diabetes Metab. J. 2015, 39,291–303.
25. International Diabetes Federation. IDFDiabetes Atlas; IDF: Watermael-Boitsfort, Belgium, 2017. Available online:http://www.diabetesatlas.org/resources/2017-atlas.html (accessed on 12 July2019).
26. Kobyliak, N.; Conte, C.; Cammarota, G.;Haley, A.P.; Štyriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P.Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab.2016, 13, 14.
27. Barrett, H.L.; Callaway, L.K.; Nitert,M.D. Probiotics: A potential role in the prevention of gestational diabetes?Acta Diabetol. 2012, 49, 1–13.
28. Mishra, A.K.; Dubey, V.; Ghosh, A.R.;Information, P.E.K.F.C. Obesity: An overview of possible role(s) of guthormones, lipid sensing and gut microbiota. Metablism 2016, 65, 48–65.
29. Zhao, X.; Higashikawa, F.; Noda, M.;Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The Obesity and Fatty LiverAre Reduced by Plant-Derived Pediococcus pentosaceus LP28 in High FatDiet-Induced Obese Mice. PLoS ONE 2012, 7, e30696.
30. Park, S.-Y.; Cho, S.-A.; Lee, M.-K.;Lim, S.-D. Effect of Lactobacillus plantarum FH185 on the Reduction ofAdipocyte Size and Gut Microbial Changes in Mice with Diet-induced Obesity.Food Sci. Anim. Resour. 2015, 35, 171–178.
31. Park, S.; Ji, Y.; Jung, H.Y.; Park, H.;Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H.Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissueaccumulation in a diet-induced obesity murine model. Appl. Microbiol.Biotechnol. 2017, 101, 1605–1614.
32. Wu, C.-C.; Weng, W.-L.; Lai, W.-L.;Tsai, H.-P.; Liu, W.-H.; Lee, M.-H.; Tsai, Y.-C. Effect of Lactobacillusplantarum Strain K21 on High-Fat Diet-Fed Obese Mice. Evid. Based Complement.Altern. Med. 2015, 2015, 1–9. [Google Scholar]
33. Park, J.E.; Oh, S.H.; Cha, Y.S.Lactobacillus plantarum LG42 isolated from gajami sik-hae decreases body andfat pad weights in diet-induced obese mice. J. Appl. Microbiol. 2014, 116,145–156.
34. Callaway, L.K.; McIntyre, H.D.;Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.;Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the Preventionof Gestational Diabetes Mellitus in Overweight and Obese Women: Findings Fromthe SPRING Double-blind Randomized Controlled Trial. Diabetes Care 2019, 42,dc182248.
35. Okesene-Gafa, K.A.M.; Li, M.; McKinlay,C.J.D.; Taylor, R.S.; Rush, E.C.; Wall, C.R.; Wilson, J.; Murphy, R.; Taylor,R.; Thompson, J.M.D.; et al. Effect of antenatal dietary interventions inmaternal obesity on pregnancy weight-gain and birthweight: Healthy Mums andBabies (HUMBA) randomized trial. Am. J. Obstet. Gynecol. 2019, 221, 1–13.
36. Krumbeck, J.A.; Rasmussen, H.E.;Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. ProbioticBifidobacterium strains and galacto oligosaccharides improve intestinal barrierfunction in obese adults but show no synergism when used together assynbiotics. Microbiome 2018, 6, 121.
37. Kim, J.; Yun, J.M.; Kim, M.K.; Kwon,O.; Cho, B. Lactobacillus gasseri BNR17 Supplementation Reduces the VisceralFat Accumulation and Waist Circumference in Obese Adults: A Randomized,Double-Blind, Placebo-Controlled Trial. J. Med. Food 2018, 21, 454–461.
38. Minami, J.; Iwabuchi, N.; Tanaka, M.;Yamauchi, K.; Xiao, J.-Z.; Abe, F.; Sakane, N. Effects of Bifidobacterium breveB-3 on body fat reductions in pre-obese adults: A randomized, double-blind,placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 67–75.
39. Ogawa, A.; Kadooka, Y.; Kato, K.;Shirouchi, B.; Sato, M. Lactobacillus gasseri SBT2055 reduces postprandial andfasting serum non-esterified fatty acid levels in Japanesehypertriacylglycerolemic subjects. Lipids Health Dis. 2014, 13, 36.
40. Dietrich, C.G.; Kottmann, T.; Alavi, M.Commercially available probiotic drinks containing Lactobacillus caseiDN-114001 reduce antibiotic-associated diarrhea. World J. Gastroenterol. 2014,20, 15837–15844.
41. Iqbal, M.Z.; Qadir, M.I.; Hussain, T.;Janbaz, K.H.; Khan, Y.H.; Ahmad, B. Review: Probiotics and their beneficialeffects against various diseases. Pak. J. Pharm. Sci. 2014, 27, 405–415.[Google Scholar]
42. Slavin, J. Fiber and Prebiotics:Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435.
43. Bejar, W.; Hamden, K.; Ben Salah, R.;Chouayekh, H. Lactobacillus plantarum TN627 significantly reduces complicationsof alloxan-induced diabetes in rats. Anaerobe 2013, 24, 4–11.
44. Sakai, T.; Taki, T.; Nakamoto, A.;Shuto, E.; Tsutsumi, R.; Toshimitsu, T.; Makino, S.; Ikegami, S. Lactobacillusplantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fatdiet. J. Nutr. Sci. Vitaminol. 2013, 59, 144–147.
45. Yakovlieva, M.; Tacheva, T.; Mihaylova,S.; Tropcheva, R.; Trifonova, K.; Tolesmall ka, C.A.; Danova, S.; Vlaykova, T.Influence of Lactobacillus brevis 15 and Lactobacillus plantarum 13 on bloodglucose and body weight in rats after high-fructose diet. Benef. Microbes 2015,6, 505–512.
46. Huang, H.-Y.; Korivi, M.; Tsai, C.-H.;Yang, J.-H.; Tsai, Y.-C. Supplementation of Lactobacillus plantarum K68 andFruit-Vegetable Ferment along with High Fat-Fructose Diet Attenuates MetabolicSyndrome in Rats with Insulin Resistance. Evid. Based Complement. Altern. Med.2013, 2013, 1–12. [Google Scholar]
47. Li, X.; Yin, B.; Fang, D.; Jiang, T.;Zhao, J.; Wang, N.; Fang, S.; Zhang, H.; Wang, G.; Chen, W. Effects ofLactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance inhigh-fat and streptozotocin-induced type 2 diabetic mice. J. Appl. Microbiol.2016, 121, 1727–1736.
48. Zuo, T.; Ng, S.C. The Gut Microbiota inthe Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front.Microbiol. 2018, 9, 2247.
49. Opazo, M.C.; Ortega-Rocha, E.M.;Coronado-Arrázola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.;Kalergis, A.M.; Riedel, C.A. Intestinal microbiota influences non-intestinalrelated autoimmune diseases. Front. Microbiol. 2018, 9, 432.
50. Jumpertz, R.; Le, D.S.; Turnbaugh,P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balancestudies reveal associations between gut microbes, caloric load, and nutrientabsorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65.
51. Ignacio, A.; Fernandes, M.; Rodrigues,V.; Groppo, F.; Cardoso, A.; Avila-Campos, M.; Nakano, V.; Avila-Campos, M.Correlation between body mass index and faecal microbiota from children. Clin.Microbiol. Infect. 2016, 22, 1–8.
52. Patil, D.P.; Dhotre, D.P.; Chavan,S.G.; Sultan, A.; Jain, D.S.; Lanjekar, V.B.; Gangawani, J.; Shah, P.S.;Todkar, J.S.; Shah, S.; et al. Molecular analysis of gut microbiota in obesityamong Indian individuals. J. Biosci. 2012, 37, 647–657.
53. Zhang, H.; DiBaise, J.K.; Zuccolo, A.;Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.;Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastricbypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370.
54. Santacruz, A.; Collado, M.C.;García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero,M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition isassociated with body weight, weight gain and biochemical parameters in pregnantwomen. Br. J. Nutr. 2010, 104, 83–92.
55. Allen, J.M.; Jaggers, R.M.; Solden,L.M.; Loman, B.R.; Davies, R.H.; Mackos, A.R.; Ladaika, C.A.; Berg, B.M.;Chichlowski, M.; Bailey, M.T. Dietary oligosaccharides attenuate stress-induceddisruptions in immune reactivity and microbial B-vitamin metabolism. FrontImmunol. 2019, 10, 1774.
56. Fei, N.; Zhao, L. An opportunisticpathogen isolated from the gut of an obese human causes obesity in germfreemice. ISME J. 2013, 7, 880–884.
57. Bervoets, L.; Van Hoorenbeeck, K.;Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.;Vankerckhoven, V. Differences in gut microbiota composition between obese andlean children: A cross-sectional study. Gut Pathog. 2013, 5, 10.
58. Duncan, S.H.; Belenguer, A.; Holtrop,G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake ofcarbohydrates by obese subjects results in decreased concentrations of butyrateand butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73,1073–1078.
59. Costa, F.R.; Françozo, M.C.; DeOliveira, G.G.; Ignacio, A.; Castoldi, A.; Zamboni, D.S.; Ramos, S.G.; Câmara,N.O.; De Zoete, M.R.; Palm, N.W.; et al. Gut microbiota translocation to thepancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset.J. Exp. Med. 2016, 213, 1223–1239.
60. Murugesan, S.; Ulloa-Martínez, M.;Martinez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.;Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Studyof the diversity and short-chain fatty acids production by the bacterialcommunity in overweight and obese Mexican children. Eur. J. Clin. Microbiol.Infect. Dis. 2015, 34, 1337–1346.
61. Verdam, F.J.; Fuentes, S.; De Jonge,C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; De Vos, W.M.;Rensen, S.S. Human intestinal microbiota composition is associated with localand systemic inflammation in obesity. Obesity 2013, 21, E607–E615.
62. Kasai, C.; Sugimoto, K.; Moritani, I.;Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; etal. Comparison of the gut microbiota composition between obese and non-obeseindividuals in a Japanese population, as analyzed by terminal restrictionfragment length polymorphism and next-generation sequencing. BMC Gastroenterol.2015, 15, 100.
63. Payahoo, L.; Khajebishak, Y.;Ostadrahimi, A. Akkermansia muciniphila bacteria: A new perspective on themanagement of obesity: An updated review. Rev. Med. Microbiol. 2019, 30, 83–89.
64. Million, M.; Angelakis, E.; Paul, M.;Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effectof Lactobacillus species on weight gain in humans and animals. Microb. Pathog.2012, 53, 100–108.
65. Qiao, Y.; Sun, J.; Xia, S.; Li, L.; Li,Y.; Wang, P.; Shi, Y.; Le, G. Effects of different Lactobacillus reuteri oninflammatory and fat storage in high-fat diet-induced obesity mice model. J.Funct. Foods 2015, 14, 424–434.
66. Ridlon, J.M.; Kang, D.J. Hylemon PB.Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006,47, 241–259.
67. Binder, H.J.; Filburn, B.; Floch, M.Bile acid inhibition of intestinal anaerobic organisms. Am. J. Clin. Nutr.1975, 28, 119–125.
68. Kurdi, P.; Kawanishi, K.; Mizutani, K.;Yokota, A. Mechanism of Growth Inhibition by Free Bile Acids in Lactobacilliand Bifidobacteria. J. Bacteriol. 2006, 188, 1979–1986.
69. Fiorucci, S.; Mencarelli, A.;Palladino, G.; Cipriani, S. Bile-acid-activated receptors: Targeting TGR5 andfarnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol. Sci.2009, 30, 570–580.
70. Thomas, C.; Gioiello, A.; Noriega, L.;Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.;Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucosehomeostasis. Cell Metab. 2009, 10, 167–177.
71. McGavigan, A.K.; Garibay, D.; Henseler,Z.M.; Chen, J.; Bettaieb, A.; Haj, F.G.; Ley, R.E.; Chouinard, M.L.; Cummings,B.P. TGR5 contributes to glucoregulatory improvements after vertical sleevegastrectomy in mice. Gut 2017, 66, 226–234.
72. Parséus, A.; Sommer, N.; Sommer, F.;Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F.Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66,429–437.
73. Flint, H.J.; Bayer, E.A.; Rincon, M.T.;Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potentialfor new insights from genomic analysis. Nat. Rev. Genet. 2008, 6, 121–131.
74. Riley, L.W.; Raphael, E.; Faerstein, E.Obesity in the United States—Dysbiosis from Exposure to Low-Dose Antibiotics?Front. Public Health 2013, 1, 69.
75. Canfora, E.E.; Meex, R.C.; Venema, K.;Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev.Endocrinol. 2019, 1.
76. Brinkworth, G.D.; Noakes, M.; Clifton,P.M.; Bird, A.R. Comparative effects of very low-carbohydrate, high-fat andhigh-carbohydrate, low-fat weight-loss diets on bowel habit and faecalshort-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101,1493.
77. Brown, A.J.; Goldsworthy, S.M.; Barnes,A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.;Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41and GPR43 are activated by propionate and other short chain carboxylic acids.J. Biol. Chem. 2003, 278, 11312–11319.
78. Xu, H.; Barnes, G.T.; Yang, Q.; Tan,G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.;et al. Chronic inflammation in fat plays a crucial role in the development ofobesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830.
79. Cani, P.D.; Amar, J.; Iglesias, M.A.;Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.;Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and InsulinResistance. Diabetes 2007, 56, 1761–1772.
80. Dalby, M.J.; Aviello, G.; Ross, A.W.;Walker, A.W.; Barrett, P.; Morgan, P.J. Diet induced obesity is independent ofmetabolic endotoxemia and TLR4 signalling, but markedly increases hypothalamicexpression of the acute phase protein, SerpinA3N. Sci. Rep. 2018, 8, 15648.
81. Amar, J.; Burcelin, R.; Ruidavets,J.B.; Cani, P.D.; Fauvel, J.; Alessi, M.C.; Chamontin, B.; Ferriéres, J. Energyintake is associated with endotoxemia in apparently healthy men. Am. J. Clin.Nutr. 2008, 87, 1219–1223.
82. Hotamisligil, G.S. Inflammation andmetabolic disorders. Nature 2006, 444, 860–867.
83. Chawla, A. Nuclear Receptors and LipidPhysiology: Opening the X-Files. Science 2001, 294, 1866–1870.
84. Glass, C.K.; Ogawa, S. Combinatorial rolesof nuclear receptors in inflammation and immunity. Nat. Rev. Immunol. 2006, 6,44–55.
85. Wellen, K.E.; Hotamisligil, G.S.Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115,1111–1119.
86. Ge, H.; Li, X.; Weiszmann, J.; Wang,P.; Baribault, H.; Chen, J.-L.; Tian, H.; Li, Y. Activation of GProtein-Coupled Receptor 43 in Adipocytes Leads to Inhibition of Lipolysis andSuppression of Plasma Free Fatty Acids. Endocrinology 2008, 149,4519–4526.
87. Semenkovich, C.F. Insulin resistanceand atherosclerosis. J. Clin. Investig. 2006, 116, 1813–1822.
88. Bäckhed, F.; Ding, H.; Wang, T.;Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gutmicrobiota as an environmental factor that regulates fat storage. Proc. Natl.Acad. Sci. USA 2004, 101, 15718–15723.
89. Shapiro, H.; Suez, J.; Elinav, E.Personalized microbiome-based approaches to metabolic syndrome management andprevention. J. Diabetes 2017, 9, 226–236.
90. Wang, Z.; Klipfell, E.; Bennett, B.J.;Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.;Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotescardiovascular disease. Nature 2011, 472, 57–63.
91. Koeth, R.A.; Wang, Z.; Levison, B.S.;Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al.Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat,promotes atherosclerosis. Nat. Med. 2013, 19, 576–585.
92. Chiang, J.Y.L. Bile acids: Regulationof synthesis. J. Lipid Res. 2009, 50, 1955–1966.
93. Vrieze, A.; Out, C.; Fuentes, S.;Jonker, L.; Reuling, I.; Kootte, R.S.; Van Nood, E.; Holleman, F.; Knaapen, M.;Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acidmetabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831.
94. Reijnders, D.; Goossens, G.H.; Hermes,G.D.; Neis, E.P.; Van Der Beek, C.M.; Most, J.; Holst, J.J.; Lenaerts, K.;Kootte, R.S.; Nieuwdorp, M.; et al. Effects of Gut Microbiota Manipulation byAntibiotics on Host Metabolism in Obese Humans: A Randomized Double-BlindPlacebo-Controlled Trial. Cell Metab. 2016, 24, 341.
95. Delgado, T.C. Glutamate and GABA inAppetite Regulation. Front. Endocrinol. 2013, 4, 103.
96. Duca, F.A.; Swartz, T.D.; Sakar, Y.;Covasa, M. Increased Oral Detection, but Decreased Intestinal Signaling forFats in Mice Lacking Gut Microbiota. PLoS ONE 2012, 7, e39748.
97. Ley, R.E.; Bäckhed, F.; Turnbaugh, P.;Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbialecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075.
98. Turnbaugh, P.J.; Gordon, J.I. The coregut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158.
99. Ussar, S.; Griffin, N.W.; Bézy, O.;Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn,C.R. Interactions between Gut Microbiota, Host Genetics and Diet Modulate thePredisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015, 22,516–530.
100. Goodrich, J.K.; Waters, J.L.; Poole,A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.;Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell2014, 159, 789–799.
101. Liu, R.; Hong, J.; Xu, X.; Feng, Q.;Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiomeand serum metabolome alterations in obesity and after weight-loss intervention.Nat. Med. 2017, 23, 859–868.
102. Bodenlos, J.S.; Schneider, K.L.;Oleski, J.; Gordon, K.; Rothschild, A.J.; Pagoto, S.L. Vagus nerve stimulationand food intake: Effect of body mass index. J. Diabetes Sci. Technol. 2014, 8,590–595.
103. Meng, F.; Han, Y.; Srisai, D.;Belakhov, V.; Farias, M.; Xu, Y.; Palmiter, R.D.; Baasov, T.; Wu, Q. Newinducible genetic method reveals critical roles of GABA in the control offeeding and metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 3645–3650.
104. Schellekens, H.; Dinan, T.G.; Cryan,J.F. Lean mean fat reducing “ghrelin” machine: Hypothalamic ghrelin and ghrelinreceptors as therapeutic targets in obesity. Neuropharmacology 2010, 58, 2–16.
105. Heisler, L.K.; Jobst, E.E.; Sutton,G.M.; Zhou, L.; Borok, E.; Thornton-Jones, Z.; Liu, H.Y.; Zigman, J.M.;Balthasar, N.; Kishi, T.; et al. Serotonin Reciprocally Regulates MelanocortinNeurons to Modulate Food Intake. Neuron 2006, 51, 239–249.
106. Xu, Y.; Jones, J.E.; Kohno, D.;Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman,J.M.; Lowell, B.B.; et al. 5-HT2CRs expressed by pro-opiomelanocortin neuronsregulate energy homeostasis. Neuron 2008, 60, 582–589.
107. Sandhu, K.V.; Sherwin, E.;Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding themicrobiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res.2016, 179, 223–244.
108. Everard, A.; Cani, P.D. Gut microbiotaand GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196.
109. Niwa, T.; Takeda, N.; Tatematsu, A.;Maeda, K. Accumulation of indoxyl sulfate, an inhibitor of drug-binding, inuremic serum as demonstrated by internal-surface reversed-phase liquidchromatography. Clin. Chem. 1988, 34, 2264–2267.
110. Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu,M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir,M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via acentral homeostatic mechanism. Nat. Commun. 2014, 5, 3611.
111. Perry, R.J.; Peng, L.; Barry, N.A.Acetate mediates a microbiome-brain-beta-cell axis to promote metabolicsyndrome. Nature 2016, 534, 213–217.
112. Swartz, T.D.; Duca, F.A.; de Wouters,T.; Sakar, Y.; Covasa, M. Up-regulation of intestinal type 1 taste receptor 3and sodium glucose luminal transporter-1 expression and increased sucroseintake in mice lacking gut microbiota. Br. J. Nutr. 2012, 107, 621–630.
113. Oh, M.S.; Phelps, K.R.; Traube, M.;Barbosa-Saldivar, J.L.; Boxhill, C.; Carroll, H.J. D-Lactic Acidosis in a Manwith the Short-Bowel Syndrome. N. Engl. J. Med. 1979, 301, 249–252.
114. Tang, W.W.; Wang, Z.; Levison, B.S.;Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbialmetabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med.2013, 368, 1575–1584.
115. Ichii, O.; Otsuka-Kanazawa, S.;Nakamura, T.; Ueno, M.; Kon, Y.; Chen, W.; Rosenberg, A.Z.; Kopp, J.B. PodocyteInjury Caused by Indoxyl Sulfate, a Uremic Toxin and Aryl-Hydrocarbon ReceptorLigand. PLoS ONE 2014, 9, e108448.
116. Vaziri, N.D.; Wong, J.; Pahl, M.;Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L.Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83,308–315.
117. Zhao, Y.-Y.; Wang, H.-L.; Cheng,X.-L.; Wei, F.; Bai, X.; Lin, R.-C.; Vaziri, N.D. Metabolomics analysis revealsthe association between lipid abnormalities and oxidative stress, inflammation,fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci.Rep. 2015, 5, 12936.
118. Chen, D.-Q.; Chen, H.; Chen, L.;Vaziri, N.D.; Wang, M.; Li, X.-R.; Zhao, Y.-Y. The link between phenotype andfatty acid metabolism in advanced chronic kidney disease. Nephrol. Dial.Transplant. 2017, 32, 1154–1166.
119. Wang, Z.; Koonen, D.; Hofker, M.; Fu,J.Y. Gut microbiome and lipid metabolism: From associations to mechanisms.Curr. Opin. Lipidol. 2016, 27, 216–224.
120. Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.;Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J.Impaired renal function and dysbiosis of gut microbiota contribute to increasedtrimethylamine-N-oxide in chronic kidney disease patients. Sci. Rep. 2017, 7,1445.
121. Vaziri, N.D.; Yuan, J.; Nazertehrani,S.; Ni, Z.; Liu, S. Chronic Kidney Disease Causes Disruption of Gastric andSmall Intestinal Epithelial Tight Junction. Am. J. Nephrol. 2013, 38,99–103.
122. Ikizler, T.A. Resolved: Being fat isgood for dialysis patients: The Godzilla effect: Pro. J. Am. Soc. Nephrol.2008, 19, 1059–1062.
123. Stenvinkel, P. Obesity in KidneyDisease. In Endocrine Disorders in Kidney Disease; Rhee, C., Kalantar-Zadeh,K., Brent, G., Eds.; Springer: Cham, Switzerland, 2019.
124. Pineiro, M.; Asp, N.-G.; Reid, G.;Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical Meeting onPrebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159.
125. Younis, K.; Ahmad, S.; Jahan, K.Health benefits and application of prebiotics in foods. J. Food Process.Technol. 2015, 6, 1.
126. Connolly, M.L.; Lovegrove, J.A.;Tuohy, K.M. In Vitro Fermentation Characteristics of Whole Grain Wheat Flakesand the Effect of Toasting on Prebiotic Potential. J. Med. Food 2012, 15,33–43.
127. Newburg, D.S. Oligosaccharides inHuman Milk and Bacterial Colonization. J. Pediatr. Gastroenterol. Nutr. 2000,30, S8–S17.
128. Everard, A.; Lazarevic, V.; Derrien,M.; Girard, M.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.;François, P.; De Vos, W.M.; et al. Responses of Gut Microbiota and Glucose andLipid Metabolism to Prebiotics in Genetic Obese and Diet-InducedLeptin-Resistant Mice. Diabetes 2011, 60, 2775–2786.
129. Cani, P.D.; Possemiers, S.; Van DeWiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.;Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota controlinflammation in obese mice through a mechanism involving GLP-2-drivenimprovement of gut permeability. Gut 2009, 58, 1091–1103.
130. Cerdó, T.; García-Santos, J.A.;Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in thePrevention and Treatment of Obesity. Nutrients 2019, 11, 635.
131. Sanders, M.E.; Merenstein, D.J.; Reid,G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal healthand disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol.2019, 16, 605–616.
132. Mishra, S.P.; Wang, S.; Nagpal, R.;Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and Prebiotics forthe Amelioration of Type 1 Diabetes: Present and Future Perspectives.Microorganisms 2019, 7, 67.
133. Hadi, A.; Mohammadi, H.; Miraghajani,M.; Ghaedi, E. Efficacy of synbiotic supplementation in patients withnonalcoholic fatty liver disease: A systematic review and meta-analysis ofclinical trials: Synbiotic supplementation and NAFLD. Crit. Rev. Food Sci.Nutr. 2019, 59, 2494–2505.
134. Sharma, R.; Kapila, R.; Kapila, S.Probiotics as Anti-immunosenescence Agents. Food Rev. Int. 2013, 29,201–216.
135. Moya-Pérez, A.; Neef, A.; Sanz, Y.Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-AssociatedInflammation by Restoring the Lymphocyte-Macrophage Balance and Gut MicrobiotaStructure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0126976.
136. Zhang, W.; Wang, H.; Liu, J.; Zhao,Y.; Gao, K.; Zhang, J. Adhesive ability means inhibition activities forLactobacillus against pathogens and S-layer protein plays an important role inadhesion. Anaerobe 2013, 22, 97–103.
137. Kowalska, I.; Straczkowski, M.;Górski, J.; Kinalska, I. The effect of fasting and physical exercise on plasmaleptin concentrations in high-fat fed rats. J. Physiol. Pharmacol. 1999, 50,309–320.
138. Gan, L.; England, E.; Yang, J.Y.;Toulme, N.; Ambati, S.; Hartzell, D.L. A 72-h high fat diet increasestranscript levels of the neuropeptide galanin in the dorsal hippocampus of therat. BMC Neurosci. 2015, 16, 51.
139. Yan, W.-J.; Mu, Y.; Yu, N.; Yi, T.-L.;Zhang, Y.; Pang, X.-L.; Cheng, D.; Yang, J. Protective effects of metformin onreproductive function in obese male rats induced by high-fat diet. J. Assist.Reprod. Genet. 2015, 32, 1097–1104.
140. Abu, M.N.; Samat, S.; Kamarapani, N.;Hussein, F.N.; Ismail, W.I.W.; Hassan, H.F. Tinospora crispa AmelioratesInsulin Resistance Induced by High Fat Diet in Wistar Rats. Evid. BasedComplement. Altern. Med. 2015, 2015, 1–6.
141. Zhou, L.; Jang, K.Y.; Moon, Y.J.;Wagle, S.; Kim, K.M.; Lee, K.B.; Park, B.-H.; Kim, J.R. Leptin amelioratesischemic necrosis of the femoral head in rats with obesity induced by ahigh-fat diet. Sci. Rep. 2015, 5, 9397.
142. Bollheimer, L.C.; Buettner, R.;Pongratz, G.; Brunner-Ploss, R.; Hechtl, C.; Banas, M.; Singler, K.; Hamer,O.W.; Stroszczynski, C.; Sieber, C.C.; et al. Sarcopenia in the aging high-fatfed rat: A pilot study for modeling sarcopenic obesity in rodents. Biogerontology2012, 13, 609–620.
143. Faragher, R.; Frasca, D.; Remarque,E.; Pawelec, G. Better immunity in later life: A position paper. AGE 2014, 36,9619.
144. Iweala, O.I.; Nagler, C.R. TheMicrobiome and Food Allergy. Annu. Rev. Immunol. 2019, 37, 377–403.
145. Yousefi, B.; Eslami, M.; Ghasemian,A.; Kokhaei, P.; Sadeghnejhad, A. Probiotics can really cure an autoimmunedisease? Gene Rep. 2019, 15, 100364.
146. Childs, C.E.; Calder, P.C.; Miles,E.A. Diet and Immune Function. Nutrients 2019, 11, 1933.
147. Yuan, Z.-P.; Yang, M.; Liang, L.; Fu,J.-F.; Xiong, F.; Liu, G.-L.; Gong, C.-X.; Luo, F.-H.; Chen, S.-K.; Zhang,D.-D.; et al. Possible role of birth weight on general and central obesity inChinese children and adolescents: A cross-sectional study. Ann. Epidemiol.2015, 25, 748–752.
148. Rogers, I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in laterlife. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 755–777.
149. Rockenbach, G.; Luft, V.C.; Mueller,N.T.; Duncan, B.B.; Stein, M.C.; Vigo, Á.; Matos, S.M.A.; Fonseca, M.J.M.;Barreto, S.M.; Benseñor, I.M.; et al. Sex-specific associations of birth weightwith measures of adiposity in mid-to-late adulthood: The Brazilian LongitudinalStudy of Adult Health (ELSA-Brasil). Int. J. Obes. 2016, 40, 1286–1291.
150. Logan, K.M.; Gale, C.; Hyde, M.J.;Santhakumaran, S.; Modi, N. Diabetes in pregnancy and infant adiposity:Systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 2017,102, F65–F72.
151. Blencowe, H.; Cousens, S.;Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Garcia,C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates ofpreterm birth rates in the year 2010 with time trends since 1990 for selectedcountries: A systematic analysis and implications. Lancet 2012, 379,2162–2172.
152. Harrison, M.S.; Goldenberg, R.L.Global burden of prematurity. Semin. Fetal Neonatal Med. 2016, 21, 74–79.
153. Lee, A.C.; Kozuki, N.; Cousens, S.;Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.;Schmiegelow, C.; Adair, L.S.; et al. Estimates of burden and consequences ofinfants born small for gestational age in low and middle income countries withINTERGROWTH-21st standard: Analysis of CHERG datasets. BMJ 2017, 358,j3677.
154. Crume, T.L.; Ogden, L.; West, N.A.;Vehik, K.S.; Scherzinger, A.; Daniels, S.; McDuffie, R.; Bischoff, K.; Hamman,R.F.; Norris, J.M.; et al. Association of exposure to diabetes in utero withadiposity and fat distribution in a multiethnic population of youth: TheExploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011,54, 87–92.
155. Malcolm, J. Through the looking glass:Gestational diabetes as a predictor of maternal and offspring long-term health.Diabetes Metab. Res. Rev. 2012, 28, 307–311.
156. Boyle, K.E.; Patinkin, Z.W.; Shapiro,A.L.B.; Baker, I.I.P.R.; Dabelea, D.; Friedman, J.E. Mesenchymal stem cellsfrom infants born to obese mothers exhibit greater potential for adipogenesis:The Healthy Start Baby BUMP Project. Diabetes 2016, 65, 647–659.
157. Whitaker, R.C.; Wright, J.A.; Pepe,M.S.; Seidel, K.D.; Dietz, W.H. Predicting obesity in young adulthood fromchildhood and parental obesity. N. Engl. J. Med. 1997, 337, 869–873.
158. Pascale, A.; Marchesi, N.; Govoni, S.;Coppola, A.; Gazzaruso, C. The role of gut microbiota in obesity, diabetesmellitus, and effect of metformin: New insights into old diseases. Curr OpinPharmacol, 2019, 49, 1–5.
159. Luoto, R.; Kalliomäki, M.; Laitinen,K.; Isolauri, E. The impact of perinatal probiotic intervention on thedevelopment of overweight and obesity: Follow-up study from birth to 10 years.Int. J. Obes. 2010, 34, 1531–1537.
160. Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim,M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp.population induced by metformin treatment improves glucose homeostasis indiet-induced obese mice. Gut 2014, 63, 727–735.
161. Vähämiko, S.; Laiho, A.; Lund, R.;Isolauri, E.; Salminen, S.; Laitinen, K. The impact of probioticsupplementation during pregnancy on DNA methylation of obesity-related genes inmothers and their children. Eur. J. Nutr. 2018, 58, 367–377.
162. Everard, A.; Belzer, C.; Geurts, L.;Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli,G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila andintestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA2013, 110, 9066–9071.
163. Spor, A.; Koren, O.; Ley, R.Unravelling the effects of the environment and host genotype on the gutmicrobiome. Nat. Rev. Genet. 2011, 9, 279–290.
164. Ursell, L.K.; Clemente, J.C.; Rideout,J.R.; Gevers, D.; Caporaso, J.G.; Knight, R. The interpersonal andintrapersonal diversity of human-associated microbiota in key body sites. J.Allergy Clin. Immunol. 2012, 129, 1204–1208.
165. Arumugam, M.; Raes, J.; Pelletier, E.;Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.;Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473,174–180.
166. Zimmer, J.; Lange, B.; Frick, J.S.;Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck,P. A vegan or vegetarian diet substantially alters the human colonic faecalmicrobiota. Eur. J. Clin. Nutr. 2012, 66, 53.
167. Salonen, A.; Lahti, L.; Salojärvi, J.;Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone,A.M.; Lobley, G.E.; et al. Impact of diet and individual variation onintestinal microbiota composition and fermentation products in obese men. ISMEJ. 2014, 8, 2218–2230.
168. Popkin, B.M. The Nutrition Transitionand Obesity in the Developing World. J. Nutr. 2001, 131, 871S–873S.
169. Caesar, R.; Tremaroli, V.;Kovatcheva-Datchary, P.; Cani, P.D.; Bäckhed, F. Crosstalk between GutMicrobiota and Dietary Lipids Aggravates WAT Inflammation through TLRSignaling. Cell Metab. 2015, 22, 658–668.
170. King, D.E. ; Mainous, AG, 3rd.Lambourne CA. Trends in dietary fiber intake in the United States, 1999–2008.J. Acad. Nutr. Diet. 2012, 112, 642–648.
171. Sonnenburg, E.D.; Sonnenburg, J.L.Starving our microbial self: The deleterious consequences of a diet deficientin microbiota-accessible carbohydrates. Cell Metab. 2014, 20, 779–786.
172. Desai, M.S.; Seekatz, A.M.;Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M. Adietary fiber-deprivedgut microbiota degrades the colonic mucus barrier and enhances pathogensusceptibility. Cell 2016, 167, 1339–1353.
173. Turnbaugh, P.J.; Hamady, M.;Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones,W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese andlean twins. Nature 2009, 457, 480.
174. Ley, R.E.; Turnbaugh, P.J.; Klein, S.;Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity.Nature 2006, 444, 1022.
175. Wu, G.D.; Chen, J.; Hoffmann, C.;Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters,W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbialenterotypes. Science 2011, 334, 105–108.
176. Guinane, C.M.; Cotter, P.D. Role ofthe gut microbiota in health and chronic gastrointestinal disease:Understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6,295–308.
177. Hildebrandt, M.A.; Hoffmann, C.;Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.; Knight, R.; Ahima,R.S.; Bushman, F.; Wu, G.D.; et al. High-fat diet determines the composition ofthe murine gut microbiome independently of obesity. Gastroenterology 2009, 137,1716–1724.
178. Zhang, Z.; Zhou, Z.; Li, Y.; Zhou, L.;Ding, Q.; Xu, L. Isolated exopolysaccharides from Lactobacillus rhamnosus GGalleviated adipogenesis mediated by TLR2 in mice. Sci. Rep. 2016, 6, 36083.
179. Kim, S.-W.; Park, K.-Y.; Kim, B.; Kim,E.; Hyun, C.-K. Lactobacillus rhamnosus GG improves insulin sensitivity andreduces adiposity in high-fat diet-fed mice through enhancement of adiponectinproduction. Biochem. Biophys. Res. Commun. 2013, 431, 258–263.
180. Savcheniuk, O.; Kobyliak, N.; Kondro,M.; Virchenko, O.; Falalyeyeva, T.; Beregova, T. Short-term periodicconsumption of multiprobiotic from childhood improves insulin sensitivity,prevents development of non-alcoholic fatty liver disease and adiposity inadult rats with glutamate-induced obesity. BMC Complement. Altern. Med. 2014,14, 247.
181. Vos, W.M. Fame and future of faecaltransplantations—developing next-generation therapies with syntheticmicrobiomes. Microb. Biotechnol. 2013, 6, 316–325.
182. Plovier, H.; Everard, A.; Druart, C.;Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.;Lichtenstein, L. A purified membrane protein from Akkermansia muciniphila orthe pasteurized bacterium improves metabolism in obese and diabetic mice. Nat.Med. 2017, 23, 107–113.
183. Depommier, C.; Everard, A.; Druart,C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter,D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila inoverweight and obese human volunteers: A proof-of-concept exploratory study.Nat. Med. 2019, 25, 1096–1103.
184. Bárcena, C.; Valdés-Mas, R.; Mayoral,P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar,N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension byfecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25,1234–1242.
185. Gough, E.; Shaikh, H.; Manges, A.R.Systematic Review of Intestinal Microbiota Transplantation (FecalBacteriotherapy) for Recurrent Clostridium difficile Infection. Clin. Infect.Dis. 2011, 53, 994–1002.
186. Available online:www.clinicaltrials.gov/ct2/results?cond=fecal+microbiota+transplantation+obesity(accessed on 12 July 2019).
187. Ridaura, V.K.; Faith, J.J.; Rey, F.E.;Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.;Bain, J.R.; et al. Gut Microbiota from Twins Discordant for Obesity ModulateMetabolism in Mice. Science 2013, 341, 1241214.
188. Cani, P.D. Human gut microbiome:Hopes, threats and promises. Gut 2018, 67, 1716–1725.
189. Roller, M.; Femia, A.P.; Caderni, G.;Rechkemmer, G.; Watzl, B. Intestinal immunity of rats with colon cancer ismodulated by oligofructose-enriched inulin combined with Lactobacillusrhamnosus and Bifidobacterium lactis. Br. J. Nutr. 2004, 92, 931–938.
190. Li, K.; Zhang, L.; Xue, J.; Yang, X.;Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulinalleviates diverse stages of type 2 diabetes mellitus via anti-inflammation andmodulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927.
191. De Filippo, C.; Cavalieri, D.; DiPaola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini,G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by acomparative study in children from Europe and rural Africa. Proc. Natl. Acad.Sci. USA 2010, 107, 14691–14696.
192. Hickl, O.; Heintz-Buschart, A.;Trautwein-Schult, A.; Hercog, R.; Bork, P.; Wilmes, P.; Becher, D. Samplepreservation and storage significantly impact taxonomic and functional profilesin metaproteomics studies of the human gut microbiome. Microorganisms 2019, 7,367.
193. Sridharan, G.V.; Choi, K.; Klemashevich,C.; Wu, C.; Prabakaran, D.; Bin Pan, L.; Steinmeyer, S.; Mueller, C.;Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and quantification ofbioactive microbiota metabolites in the mouse gut. Nat. Commun. 2014, 5, 5492.
原文链接:https://www.mdpi.com/2076-2607/7/10/456/htm#B66-microorganisms-07-00456
作者|Kaliyan Barathikannan 等人
翻译|gemiu
审校|617